Abstract
Introduction: Outcomes of patients with post-MPN AML remain poor with no available standard therapy. The Janus kinase 1/2 (JAK1/2) inhibitor ruxolitinib (RUX) markedly improves symptoms and splenomegaly and extends overall survival (OS) in patients with myelofibrosis (MF). In a phase 2 study of RUX in pts with relapsed/refractory (R/R) leukemia, 3 of 18 pts with post-MPN AML achieved a complete response (CR) with or without count recovery and RUX was well tolerated up to 50 mg twice daily (bid). The hypomethylating agent (HMA) decitabine (DAC) is widely used for the treatment of AML. HMAs have significant activity in post-MPN AML. The combination of RUX and DAC may target distinct clinical and pathological manifestations of post-MPN AML.
Methods: For phase I, patients with R/R AML were eligible irrespective of history of antecedent MPN. Phase II enrollment is limited to pts >60 years with post-MPN AML. DAC was administered at 20 mg/m2/d for 5 days in 4-6 weeks cycles and RUX was given uninterrupted starting day 1 of DAC. In the phase I portion, the RUX dose was escalated in "3+3" fashion from 10 through 50 mg bid (dose levels 0, 1, 2 and 3 = 10, 15, 25 and 50 mg bid, respectively). Dose limiting toxicities (DLTs) were defined in the first cycle. Response assessment is performed according to published criteria for AML. Pts can receive up to 24 cycles of therapy and may proceed to allogeneic stem cell transplantation (alloSCT) at any time following cycle 1. Measurement of circulating cytokine levels, intracellular signaling proteins and gene methylation assays were performed after cycles 1 and 3 and every 3 cycles thereafter.
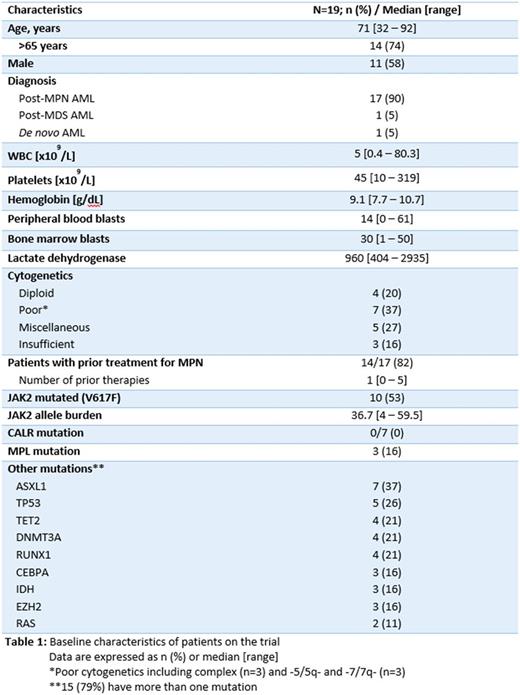
Results: A total of 19 pts (Phase I=14, phase II=5) have been enrolled (Table 1). The median age was 71 (range, 32-92) and 11 (58%) were male. The median bone marrow blast percentage was 30 (range, 1-50). Ten pts (53%) had J AK2V617F mutation. Cytogenetics (CG) were poor-risk in 7 (37%), diploid in 4 (20%) and miscellaneous in 5 (27%) pts. All but 2 pts had post-MPN AML (n=17). For pts with post-MPN AML (n=17), 14 (82%) had prior therapy for their MPN (median 1; range 0-5), including hydroxyurea alone (n=4) and RUX alone (n=2), while 7 had multiple treatments and 4 had no prior therapy. The phase I portion of the study has completed accrual. Of the 14 patients treated in phase I, 2 did not complete 1 cycle of treatment and hence were not evaluable for DLT (withdrew consent). Most adverse events (AEs) were grades 1/2. Grade 3/4 AEs included febrile neutropenia, myelosuppression, fracture, fatigue, anorexia, muscle weakness, confusion, ileus, and transaminitis; of these, only grade 3/4 thrombocytopenia and neutropenia were judged possibly related to the therapy. No DLTs occurred and the recommended phase 2 dose (RP2D) was RUX 50 mg bid with standard dose DAC. Overall, the median number of cycles received was 2 (range, 1-7) and treatment is currently ongoing in 3 pts in phase II. The most common reasons for ending study therapy were disease progression [n=8 (42%) all in phase I] and lung cancer diagnosed shortly after starting DAC+RUX (phase II; therefore inevaluable for response). Two patients died during study of pneumonia and 10 at later follow-up from infectious complications (n=9) and gastric hemorrhage (n=1). The median overall survival for patients on study was 8 months (range, 1-20). CR (n=1) and CR with incomplete counts recovery (CRi; n=3) as best responses were seen in 4/14 (29%) evaluable patients; one had TP53 mutation and complex CG. One patient in CR was bridged to SCT.
Conclusion: The combination of RUX and DAC is tolerable and shows significant efficacy in post-MPN AML with CR+CRi of 29%. The recommended phase 2 dose is 50 mg bid of RUX and 20 mg/m2/d of DAC for 5 days and the study is continuing accrual.
Bose: Incyte Corporation: Honoraria. Verstovsek: Lilly Oncology: Research Funding; Seattle Genetics: Research Funding; Roche: Research Funding; Genentech: Research Funding; Bristol Myers Squibb: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; NS Pharma: Research Funding; Lilly Oncology: Research Funding; Promedior: Research Funding; Promedior: Research Funding; Pfizer: Research Funding; Pfizer: Research Funding; Celgene: Research Funding; Roche: Research Funding; Gilead: Research Funding; Astrazeneca: Research Funding; CTI BioPharma Corp: Research Funding; Incyte: Research Funding; Astrazeneca: Research Funding; Galena BioPharma: Research Funding; NS Pharma: Research Funding; Gilead: Research Funding; Bristol Myers Squibb: Research Funding; CTI BioPharma Corp: Research Funding; Celgene: Research Funding; Galena BioPharma: Research Funding; Blueprint Medicines Corp: Research Funding; Blueprint Medicines Corp: Research Funding; Genentech: Research Funding. Jain: Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Abbvie: Research Funding; Verastem: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. DiNardo: Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Pemmaraju: Incyte: Consultancy, Honoraria; Cellectis: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Stemline: Consultancy, Honoraria, Research Funding; LFB: Consultancy, Honoraria, Research Funding. Daver: Karyopharm: Consultancy, Research Funding; Kiromic: Research Funding; Immunogen: Research Funding; Bristol-Myers Squibb Company: Consultancy, Research Funding; Otsuka America Pharmaceutical, Inc.: Consultancy; Daiichi-Sankyo: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Pfizer Inc.: Consultancy, Research Funding; Jazz: Consultancy; Incyte Corporation: Honoraria, Research Funding. Kantarjian: Novartis: Research Funding; Bristol-Meyers Squibb: Research Funding; Pfizer: Research Funding; Delta-Fly Pharma: Research Funding; ARIAD: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal